What Is Matter? Understanding Structure and Properties of Matter
According to definition, the subject Chemistry is the study of matter, its composition and the changes it undergoes. Before we start exploring the fascinating aspects of chemistry, let us start by understanding what matter is.
Explanation of Matter
Matter is anything that has mass and occupies space. Matter can exist in different forms or states and has various characteristics. Also, any object that has a physical form can be made of matter. Tiny bits of atoms are bound together to form molecules, that makeup matter. Your table, the chair you are sitting on, your food, all of this is matter.
Three States of Matter
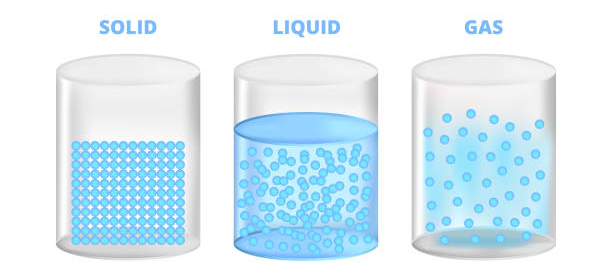
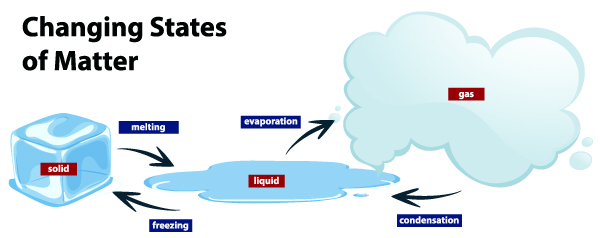
There are 3 distinct states of matter; solid, liquid, and gases. Solids have fixed shape and volume- ex: a rock. Liquids have a fixed volume, but take up the shape of the container ex: water. Gases have no volume and no shape ex: air in vehicle tires.
What is a substance?
Substance is a form of matter that contains only one type of atom or molecule. That means substance is matter in which all parts are alike. Substance is further divided into two sub-categories:
Elements
An element is a chemical substance that is made up of a piacular kind of atom and cannot be broken down further or transformed by a chemical reaction into different elements. Examples of elements are; gold, oxygen, sulfur, and iron.
- Compounds
Compounds on the other hand; are pure substances that are made up of two or more elements. Examples of compounds are; salt, water, and sugar.
Mixtures
A mixture is formed when two or more substances are mixed together. A Mixture can either have homogenous or heterogeneous properties. Let us find out what this means;
- Homogenous Mixture
In a homogenous mixture, the components mix with each other having a uniform composition. However, the components cannot be seen with the naked eye. Also, the components cannot be separated from the mixture easily. Example: salt solution, sugar solution, and detergent.
- Heterogenous Mixture
A heterogeneous mixture is one, in which the composition is not uniform throughout and different components are observed. Unlike Homogenous mixtures, components of Heterogenous mixtures as visible with the naked eye. Even the size of the particles are large. You can separate the components of a heterogeneous mixture. Example: salt and oil, ice cubes in a drink, sand & water, etc.
Methods for Separating Mixtures
The components of a mixture can be separated physically. However, the technique to be used may be different for different types of mixtures and their chemical composition. Here are the different ways in which mixtures can be separated;
- Using a Separating Funnel
A separating funnel can be used to separate two immiscible liquids. These are those liquids that do not mix together, instead, they form different layers that can be clearly seen with the naked eye. Examples of immiscible liquids are; kerosene and water, oil and water, honey and oil, etc.
Experiment:
Fill the separating funnel with kerosene and water and allow it to settle down. You will clearly see two distinctive layers forming; water is at the bottom layer & kerosene at the top. Open the stopcock of the separating funnel. You will notice that water flows out onto a different vessel. When all the water is emptied, shut the stopcock, so that the kerosene remains in the separating funnel.
Evaporation
Through evaporation, you can separate solids that are dissolved in water. The thought behind this is, solids do not evaporate, but liquids do, and the solid substance is left behind in the vessel.
- Experiment
Take a glass and mix water with salt. Now empty the salt & water mixtures into a petri dish and heat is continuously over a burner. By heating, the water starts to form vapor and evaporates into the air. Keep heating until all the water is evaporated and only the salt remains in the dish.
Distillation
Distillation is the process of separating the components or substances from a liquid mixture, by using the technique of boiling and condensation.
- Experiment
Add a few drops of ink into water and empty it into a Conical flask. Add some anti-bumping granules in the conical flask and arrange the apparatus and test tubes, before you begin with the experiment. Heat the conical flask over medium flame using a burner, and watch the ink as it starts to boil. Observe the delivery tube and notice condensation starting to happen, as vapor turns into liquid again. Wait for all the water to condense, leaving only the liquid ink in the flask.
Carolina’s Building Blocks of Science® 3D: Structure and Properties of Matter (©2019) Carolina is a world-renowned supplier of Science lab equipment to schools across the globe and in Dubai, Middle East. The chemistry module on ‘Structure and Properties of Matter’ Is aimed at students of Grade 5 and it helps students discover the physical and chemical properties of matter. The kit comprises all the materials to conduct experiments, a teacher’s guide, and a student reader.






Recent Comments